SEASONAL FLU VACCINES, ARE THEY SAFE OR NECESSARY?
By Mary Tocco
August 2, 2011
NewsWithViews.com
The seasonal flu vaccine is now recommended every year for every man, woman and child from the age of 2 throughout the rest of life. This mandate is the result of the exaggerated H1N1 Flu pandemic of 2009 that proved to not even be a pandemic. This is a political vaccine with no scientific basis that will make millions for the flu manufactures and cause health problems for thousands. How does a person sift between the conflicting information provided? As a 30+ year independent vaccine researcher, I hope these facts will help you decide if you or your children will receive the seasonal flu vaccine.
The first H1N1 flu vaccine of 2009 has proven to cause many unwanted side effect. Between the Vaccine Adverse Events Reporting System (VAERS) in Washington and other reliable sources, the side effects are stacking up and looking more like a horror film. Not only was the vaccine rushed to the market, it was based on flawed reports from the Center of Disease Control and the World Health Organization.
It is common knowledge that the flu vaccines have always had the potential to cause serious side effects. Each year the pharmaceutical companies release new flu shots that are virtually untested. They combine various flu virus strains based on an educated guess and then recommend the shot to everyone, including children and pregnant mothers. According to the CDC Vital Statistics Report 1999-2003, Influenza death for children under the age of 5 skyrocketed as they began to implement the flu vaccine for the children. From 1999 to early 2002, death rates were declining from 25 down to 10 per year then the latter half of 2002 the CDC mandated the flu vaccine for children and the death rate climbed from 25 deaths per year in 1999 to over 90 in 2002! Death is a pretty bad vaccine side effect!
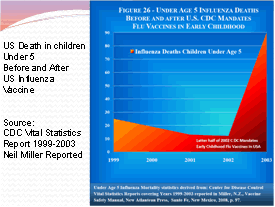
1999
— 29 deaths
2000 — 19 deaths
2001 — 13 deaths
2002 — 12 deaths
2003 — 90 deaths (Year of mass vaccinations of children under
age 5 years)
2006 — 78 deaths
2007 — 88 deaths
2008 — 116 deaths (40.9% vaccinated at age 6 months to 23 months)
What other side effects did we see with the H1N1 flu shot in 2009-2010? According to the Vaccine Adverse Events Reporting System (VAERS), there were 178 miscarriages after mothers received the H1N1 flu vaccine and 70 other documented form reliable sources. Considering that only 10% of all adverse events get reported, we know that the true numbers were much worse. According to testimony before the CDC advisory Commission on Childhood Vaccines (ACCV), Thursday, October 28, 2010 presented by Dr. Renee Tocco, on behalf of the National Coalition of Organized Women, (NCOW) the number of miscarriages we as high as 3,587 nationwide. Miscarriages, a pretty bad vaccine side effect! Dr. Renee went on to testify that the H1N1 flu pandemic was based on false information from the Center of Disease Control and the World Health Organization. They claimed the pandemic in April 2009 was based on 56 maternal deaths saying it was a “Never before seen virus”. (This virus was in three flu vaccines given to thousands of people from 2006 forward: FluMist, Focetria (swine flu) and Fluvarin all contain the H1N1 virus.) They also stated the following: “In spite of the 178 VAERS fetal-death-associated influenza vaccine reports, the FDA has approved seasonal flu vaccines for the 2010-2011 flu season that, in addition to another “A” strain and a “B” strain of influenza, contain the “same” level of the “same” 2009-A-H1N1 viruses that were present in the 2009-2010 pandemic “swine flu” vaccines and has again approved several Thimerosal-preserved flu-shot formulation that may be given to pregnant women without a prominent “Warning: Contains Mercury” caution on the vial.” It is very clear that the Center for Disease Control is not about protecting people but focused on pushing unsafe vaccines on the unsuspecting public.
In 2010, Dr. Alicia Siston studied the 56 women who died supposedly from the H1N1 flu. Her study was referenced at this hearing, showing that most of these deaths were “Unconfirmed” as being H1N1 related deaths despite the fact that the CDC had tests that could have verified for certain that these were H1N1 related. The CDC used the death of these women to push their agenda...flu vaccines for every American, without sufficient cause.
Every vaccine has risks, even the manufactures of vaccines admit that some will die and get injured. Unfortunately for the injured or the families of the dead, the manufactures are completely protected from all liability. The following is from the vaccine package insert admitting that it can be dangerous for many, untested for safety in pregnancy and no studies showing how it may affect the nursing baby:
Section 8- This medication should not be used if you have certain medical conditions. Before using this medicine, consult your doctor or pharmacist if you have: history of allergy to egg or egg products, immune deficiency (e.g., agammaglobulinemia, HIV infection, leukemia, lymphoma, other cancers, radiation). Also avoid close contact with people who are immune-compromised (e.g., HIV infection, cancer therapy) for at least 21 days. This medication is not recommended for use during pregnancy. It is also not known whether FLUVIRIN® or FluZone can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. It is not known whether this drug passes into breast milk. Consult your doctor before breast-feeding.
How is it that they offer the flu vaccine at the airport, local drug stores, grocery store pharmacies and many other places where they do not have your medical rerecords? Are the doctors telling the pregnant women that your unborn child may be at risk? Are they telling Mom that the vaccine may also contain (Thimerosal) that can potentially harm the unborn developing brain and be dangerous for mother too? What happens if the Thimerosal passes into the breast milk of nursing babies?
Another huge problem is that all major hospitals and medical facilities are now requiring that all employees get the flu shots or get fired from their jobs! Imagine working as a nurse for 25 years only to be told that you no longer have the right to choose what drug you must take to keep your job. This is a direct violation of personal rights and over rides many state laws that give people the right to choose. It will not stop there…soon it will be the DPT (diphtheria, pertussis and tetanus) vaccine because of the pertussis outbreaks across the country. What vaccine will it be next, the cancer or AIDS vaccine? I am encouraging nurses and doctors to unite and fight these unlawful mandates. Perhaps when we have numerous law suits in the courts, they will stop acting like the medical mafia.
You now must decide who you are going to trust for your information and health care recommendations. I encourage all people to thoroughly investigate all medications and vaccines before they agree to inject them. I know it can be very confusing as conflicting information is everywhere. Ultimately, it is your right to choose what your family does.
As a 30 vaccine investigator, I know the dedication and time involved to make informed vaccine decisions. I personally no longer trust the CDC, the FDA and the current medical establishment who recommend routine vaccines for all people. They have failed to protect our families and no longer represent the people. This industry is fueled by greed, special interest groups and unfortunately our government is controlled by the big pharmaceutical companies.
Flu Mist: The nasal vaccine has never been studied to see if the viruses can penetrate the membrane between the sinus and the brain (barrier). When the CDC was asked if this had been studied by Dr. Mark Geier, their response was “NO.” Common Flu Vaccine Ingredients include:
•
Egg protein – causes egg allergies
• Formaldehyde- Formalyn (formalin) is a 37 percent
solution of gaseous formaldehyde which includes methanol. Known toxin
used in embalming
• Polysorbate 80 shown to cause infertility in
mice
• Sodium Chloride and Calcium Chloride
• Monosodium Glutamate (MSG):C5H8NNaO4, a Stabilizer
MSG – man made exciito-toxin
• Potassium phosphate- a soluble salt
which is used as a fertilizer,
a food
additive and a fungicide.
• Thimerosal a form of mercury still found in
some multi-vile vaccines.
• Polyoxidonium- Synthetic polymers and nanomaterials
display selective phenotypic effects in cells and in the body that affect
signal transduction mechanisms involved in inflammation, differentiation,
proliferation, and apoptosis.
• Squalene- An oil based adjuvant that has never
been approved in the US as safe, can cause blindness, autoimmune dysfunction
and can inhibit sperm production. More than two dozen peer-reviewed
scientific papers from ten different laboratories throughout the U.S.,
Europe, Asia, and Australia have been published documenting the development
of autoimmune disease in animals subjected to squalene-based adjuvants.
Novartis will make a flu vaccine using MF59 consisting of squalene.
• Tween 80 - A recent study (December 2005) discovered
that Tween80 can cause anaphylaxis, a sometimes fatal reaction characterized
by a sharp drop in blood pressure, hives, and breathing difficulties.
• Human Diploid Tissue- organ and tissue from
aborted baby tissue is now used in manufacturing many vaccines.
Contraindications for administering the various Flu vaccines the manufactures package insert are dismissed. The package inserts claim the following:
MedImmune - Fluzone
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Fluzone and Fluzone High-Dose
Pregnancy Category C: Animal reproduction studies have not been conducted with Fluzone or Fluzone High-Dose. It is also not known whether Fluzone or Fluzone High-Dose can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Fluzone or Fluzone High-Dose should be given to a pregnant woman only if clearly needed.
Fluzone Intradermal
Pregnancy Category B: A developmental and reproductive toxicity study has been performed in female rabbits at a dose approximately 20 times the human dose (on a mg/kg basis) and has revealed no evidence of impaired female fertility or harm to the fetus due to Fluzone Intradermal. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, Fluzone Intradermal should be used during pregnancy only if clearly needed. Healthcare providers are encouraged to register women who receive Fluzone Intradermal during pregnancy in Sanofi Pasteur Inc.'s vaccination pregnancy registry by calling 1-800-822-2463.
8.3. Nursing Mothers
It is not known whether Fluzone or Fluzone Intradermal is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Fluzone or Fluzone Intradermal is administered to a nursing woman.
8.4. Pediatric Use
Fluzone
Safety and effectiveness of Fluzone in children below the age of 6 months have not been established. Safety and immunogenicity of Fluzone was evaluated in children 6 months through 8 years of age. [See Adverse Reactions (6.1) and Clinical Studies (14.1).]
Fluzone High-Dose
Safety and effectiveness of Fluzone High-Dose in persons <65 years of age have not been established.
Fluzone Intradermal
Safety and effectiveness of Fluzone Intradermal in persons <18 years of age have not been established. In a clinical trial, 97 infants and toddlers 6 months through 35 months of age and 160 children 3 years through 8 years of age were enrolled to receive two injections of Fluzone Intradermal. Infants and children in a control group received two injections of Fluzone. Fluzone Intradermal was associated with increased local reactogenicity relative to Fluzone. The size of the study was not adequate to reliably evaluate serious adverse events or the immune response elicited by Fluzone Intradermal relative to Fluzone.
8.5. Geriatric Use
Fluzone
In two observational studies of Fluzone in 118 adults 19 through 59 year
Flu Mist – MedImmune- Here are some points of interest taken from the package insert:
5.7 Limitations of Vaccine Effectiveness- FluMist may not protect all individuals receiving the vaccine.
8.1 Pregnancy
Pregnancy Category C
Animal
reproduction studies have not been conducted with FluMist. It is not
known whether FluMist can cause fetal harm when administered to a pregnant
woman or can affect reproduction capacity. FluMist should be given to
a pregnant woman only if clearly needed. The effect of the vaccine on
embryo-fetal and pre-weaning development was evaluated in a developmental
toxicity study using pregnant rats receiving the frozen formulation.
Groups of animals were administered the vaccine either once (during
the period of organogenesis on gestation day 6) or twice (prior to gestation
and during the period of organogenesis on gestation day 6), 250 microliter/rat/occasion
(approximately 110-140 human dose equivalents), by intranasal instillation.
No adverse effects on
pregnancy, parturition, lactation, embryo-fetal or pre-weaning development
were observed. There were no vaccine-related fetal malformations or
other evidence of teratogenesis noted in this study.
8.3 Nursing Mothers
It is not known whether FluMist is excreted in human milk. Therefore, as some viruses are excreted in human milk, caution should be exercised if FluMist is administered to nursing mothers.
8.4 Pediatric Use
|
|
Safety and effectiveness of the vaccine has been demonstrated for children 2 years of age and older with reduction in culture-confirmed influenza rates compared to active control (injectable influenza vaccine made by Sanofi Pasteur Inc.) and placebo [see Clinical Studies (14.1)]. FluMist is not approved for use in children — 24 months of age. FluMist use in children — 24 months has been associated with increased risk of hospitalization and wheezing in clinical trials [see Warnings and Precautions (5.1) and AdverseReactions (6.1)].













 Share
This Article
Share
This Article



